Last Updated: February 2020 by Narasimhan Santhanam
This pos![]() t is a part of EV Next’s EV Perspectives.
t is a part of EV Next’s EV Perspectives.
EV Next, a division of EAI, is a leading market intelligence & strategic consulting firm for the Indian e-mobility sector.
Get to know about 1000+ EV innovations from EVI2: Electric Vehicle Innovation Intelligence from EVNext
Introduction
Batteries are one of the major and costly components of an Electric Vehicle (EV). With the development of EV industry the battery chemistry has also evolved towards betterment. During initial days the Electric vehicles were mainly powered by lead acid batteries. This technology was widely used at that time because they are readily available and are easy to manufacture. But they have some major downsides like its weight, low energy density, discharge capacity, shorter life span and toxicity etc. This made the Nickel-Metal hydride chemistry popular as they had twice the energy density of lead acid chemistry, harm less, recyclable, high cycle life, higher operational temperature range and resistance to discharge and overcharge.
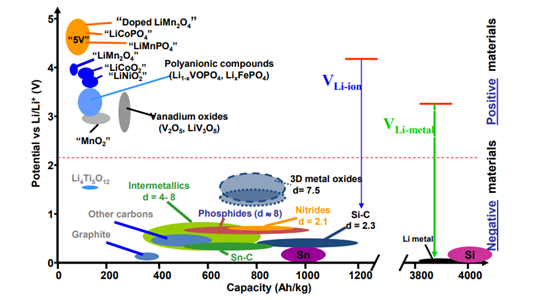
Now the industry has completely moved towards Lithium-ion batteries although the cheaper e bikes in India still use lead acid batteries to save cost. Lithium ion batteries are more common because they are the most energetic rechargeable batteries available. They can store more energy per kilogram as compared to lead acid or Ni-Mh based batteries.The graph shows the limitation of cathode material, as the capacity is not going beyond 200Ah/kg
- NMC: Provides high capacity and high power. Serves as Hybrid Cell. Favorite chemistry. For many uses market share is increasing. Slowly replacing LFP Lithium batteries
- LFP: Very flat discharge characteristic. Relatively higher self discharge rate. One of the safe lithium ion chemistry. China is a larger producer of this battery
- NCA: Best Compromise of power and energy density. Voltage (single slope) is an indication of state of charge (SOC).
- Out of NMC, LFP & NCA, NMC is a better selection for batteries because of high capacity & high power whereas LFP have specific energy between 90-120(Wh/ kg) & NCA is cost effective but have safety issues because of thermal runaway
| Cell Chemistry | LFP/Graphite | NMC/Graphite | NCA/Graphite |
| Chemical Formula | LiFePO4 | LiNi1/3Mn1/3Co1/3O2 | LiNi0.8Mn0.1Co0.05O2 |
| Nominal Voltage(V) | 3.2 | 3.7 | 3.7 |
| Operating Voltage (V) | 2.5-3.5 | 3-4.2 | 3-4.2 |
| Specific energy (WH/Kg) | 90-120 | 150-200 | 150-200 |
| Max C Rate | Up to 2C | 3C | 2C |
| Cycle Life | 2000-3000 | 2000 | 2000 |
| Thermal Runaway | 270˚ (Relatively safe) | 120˚ (High charge rate, Overcharge leads to thermal run away) | 150˚ (High charge rate, Overcharge leads to thermal run away) |
| Average Module Price($/kWh) | 270 | 400 | 230 |
Source: CEIR- CECRI
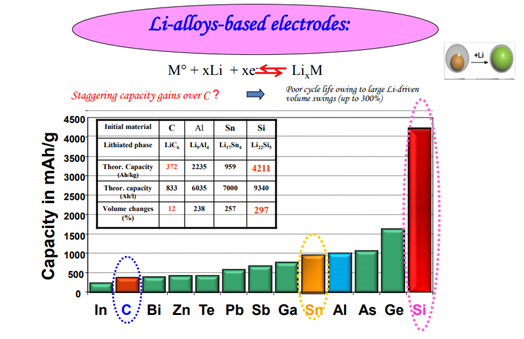
In case of Anode market have moved from Carbon to Si with a high capacity of 4000 Ah/kg but making it nano is an issue here
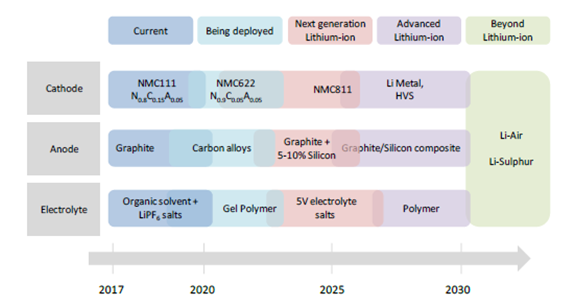
Expected battery technology commercialisation timeline
Indications from recent assessments of battery technologies suggest that lithium-ion is expected to remain the technology of choice for the next decade. The main developments in cell technology that are likely to be deployed in the next few years include:
For the cathode, the reduction of cobalt content in existing cathode chemistries, aiming to reduce cost and increase energy density, i.e. from today’s NMC 111 to NMC 622 by 2020, or from the 80% nickel and 15% cobalt of current NCA batteries to higher shares of nickel
For the anode, further improvement to the graphite structure, enabling faster charging rates
For the electrolyte, the development of gel-like electrolyte material. The next generation of Li-ion batteries entering the mass production market around 2025 is expected to have low cobalt content, high energy density and NMC 811 cathodes. Silicon can be added in small quantities to the graphite anode to increase energy density by up to 50%, while electrolyte salts able to withstand higher voltages will also contribute to better performance.
In the 2025-30 periods, technologies that promise significantly higher energy densities are likely to begin entering the market and would push the limits of Li-ion batteries (advanced Li-ion). For example, lithium metal cathodes are a promising avenue for Li-ion batteries with improved performance without relying on cobalt and anodes made of silicon composite might enter the design. In this period, solid state electrolytes might also be introduced and further improve energy density and battery safety.
The Li-ion technology might be overtaken by other battery designs that boast higher theoretical energy densities as well as lower theoretical costs. Examples include Li-air and Li-sulphur batteries. However, their technology readiness level is very low, practical performance has yet to be tested and the performance advantage over lithium-ion is still unproven. Even if battery cells with substantially different designs were to become available in the market by 2030, a time lag due to the need to build up production capacity would delay wide availability on the market for these advanced technologies. This is why most batteries are expected to belong to the “Next generation” technology class in 2030.
Source: CSIR-CECRI
Lithium-ion is expected to remain the technology of choice for the next decade, when it is expected to take advantage of a number of improvements to enhance battery performance. Other technology options are expected to become available after 2030.
Chemistry of Cells produced by major battery cell manufacturers for different EV maker
| Cell Manufacturer | Anode/ Cathode Chemistry | Capacity(Ah) | Cell Voltage (V) | Energy density(Wh/Kg) | EV Model |
| AESC | G/LMO-NCA | 33 | 3.75 | 155 | Nissan/Leaf |
| LG Chem | G/NMC-LMO | 36 | 3.75 | 157 | Renault/ ZOE |
| Panasonic | G/NCA | 3.1 | 3.6 | 265 | Tesla/X,S,3 |
| Toshiba | LTO/NMC | 20 | 2.3 | 89 | Honda/Fit |
| Samsung | G/NMC-LMO | 64 | 3.7 | 132 | Fiat/500 |
| Li Energy Japan | G/LMO-NMC | 50 | 3.7 | 109 | Mitsu-bishi/i-MeV |
- The anode (or negative electrode) in Lithium-ion battery is typically made up of Graphite, coated on Copper Foil. Graphite is a crystalline solid with a black/grey color and a metallic sheen. Due to its electronic structure, it is highly conductive and can reach 25,000 S/cm2 in the plane of a single-crystal.
- Graphite is commonly used as the active material in negative electrodes mainly because it can reversibly place Lithium-ions between its many layers. This reversible electrochemical capability is maintained over several thousands of cycles in batteries with optimized electrodes. However, one requirement for this application is that the Graphite surface must be compatible with Lithium-ion battery chemistry (salts, solvents and binders).
- Other common Anode materials that have the potential to replace graphite are Carbon alloys and Silicone composite. In some cases graphene with a small percentage of silicon is also used as anode.
2020-2030 Trends
The anode is worth 10–15% of the total cost of a lithium-ion battery, according to Chloe Holzinger, an energy storage analyst with Lux Research. The global anode material market could be worth $10 billion by 2025.
Graphite is the primary anode material
- Graphite is the most commonly used material for EV battery anodes. 25kg of high purity graphite is needed for an average-sized battery, and up to 54kg for large batteries such as those used in the Tesla Model S.
- Producing anode-grade graphite with 99.99 percent purity is expensive and the process creates waste. The end-cost is not so much the material but the purification process. Recycling old Li-ion to retrieve graphite will not solve this because of the tedious purification process
- Graphite comes in two forms: natural graphite from mines and synthetic graphite from petroleum coke. Both types are used for Li-ion anode material with 55 percent gravitating towards synthetic and the balance to natural graphite. Synthetic graphite for Li-ion sells for around US $10,000 per ton whereas spherical graphite made from natural flake sells for US $7,000 (2015 prices)
- Artificial graphite in battery anodes is expected to nearly double by 2025, to 320,000 tonnes from 165,000 tonnes in 2020
Silicon Replacing Graphite in various proportions
- Silicon has a number of advantages over graphite as an anode material, including the lower cost of the material and manufacturing. Also, it can absorb and contain a much higher number of lithium ions upon charging than graphite. This increases the efficiency of the battery, meaning EVs can reach higher distances on a single charge. Silicon anodes are still in development, but it’s likely that they will be in commercial use by 2020.
- Sila Nanotechnologies in 2011 to develop a commercial silicon anode. The conventional wisdom is to replace, say, 10% of the graphite in a battery anode with silicon metal or oxide, improving density without introducing too much swelling. The company has created a nanocomposite of covalently bonded nanostructures of which 50% are silicon and the rest undisclosed non graphite materials. The composite is porous but encapsulated with a sealed outer layer that prevents electrolyte penetration into the composite, protecting it from damage during charge and discharge. The composite is contained in a porous scaffold structure so it is able to expand and contract without puncturing the coating. Sila’s material has an energy storage capacity four or five times that of graphite, enabling the energy density of a lithium-ion battery to increase by 20–40%.
- Amprius has a 100% silicon anode that Airbus successfully tested in lithium-ion batteries for its Zephyr S pseudo satellite. The batteries have an energy density of over 435 (W h)/kg—substantially higher than that of commercial lithium-ion batteries in use today.
Lithium metal Anode
- A lithium-metal anode has the highest specific capacity of any anode material, at 3,862 (mA h)/g, says Oxis’s chief technical officer, David A. Ainsworth. When paired with an optimized sulfur-based cathode, it will allow Oxis’s Li-S battery to achieve an energy density of more than 425 (W h)/kg, he says, compared with about 200 (W h)/kg for a lithium-ion battery.
- Lithium-sulfur batteries are known to suffer from fading performance after a number of recharge cycles.
- Equipping the battery with a lithium sulfide electrolyte protects the lithium-sulfide anode from degradation because the electrolyte instantly forms a film on the anode With a melting point of more than 900 °C, this coating protects the lithium even at extreme temperatures.( information from Oxis Company)
- Volta Energy’s Chamberlain says. The most likely scenario will be the gradual adoption of silicon blended with graphite in anodes, rather than a jump to 100% silicon or lithium, he says. Overshadowing these marketplace developments is uncertainty about intellectual property rights relating to silicon anode technology because so many patents have been filed. About 1,100 patents relating to silicon anodes were filed in 2016 alone, and filings are increasing annually, according to the technology market research firm IDTechEx. Samsung was the most prolific in 2016 with almost 250 patents filed, followed by LG Chem, Panasonic, Sony, and Nexeon.
Lithium Titanate Oxide (lithium titanium oxide) Battery Technology
- Lithium titanate (LTO) replaces the graphite in the anode of a standard lithium-ion battery and the material forms into a spinel structure. It can be used in combination with LMO or NMC cathode
- In essence, the LTO is a rechargeable battery based on the, or modified from, the Lithium-Ion (li-ion) battery technology. Li-titanate oxide (LTO) replaces the graphite in the anode of the typical Li-Ion battery and forms the materials into a spinel 3D crystal structure. Having a nominal a cell voltage of 2.40V, it releases a high current discharge current that is 10 times the capacity of the other types of lithium batteries. Instead of using carbon particles on its surface as other lithium batteries do, Lithium Titanate utilizes lithium-titanate nanocrystals.
- The effect and benefit of this alteration and inclusion of lithium-titanate nanocrystals is that the surface area of the anode of the Lithium-Titanate battery is about 100 square meters per gram in contrast to the only 3 square meters per gram that Li-Ion batteries hold. The result of the lithium-titanate nanocrystals with their enlarged surface area is that electrons are able to enter and leave the anode much more rapidly, leading to fast recharging and enhanced lifetimes of the battery.
Source: Battery University (https://batteryuniversity.com/)
Read more on the EV Battery ecosystem from: EV battery Innovations | Components of BMS | FCEV Trends | FCEV Indian Efforts | Anode/Cathode R&D | Li-ion Battery Trends | BMS Innovations | Indian Battery Manufacturers | Cost of Li-ion Batteries | Anode Materials in 2020-2030 | Key Drivers shaping Battery Chemistry |
![]() Know more on how EV Next can assist your business in your strategy for the e-mobility and electric vehicles sectors, Here
Know more on how EV Next can assist your business in your strategy for the e-mobility and electric vehicles sectors, Here
Wish to know everything about India’s EV market from one place? Check out the India EV Expert Guide, an 800 page comprehensive guide to the Indian EV market. Here
Get to know about 1000+ EV innovations from EVI2: Electric Vehicle Innovation Intelligence from EVNext
See also the blog posts:
- Innovations in Battery Management System – BMS Technology Trends – Charging, Intelligence, Analytics
- Technologies and Innovations in EV Industry – Better Batteries, Lighter Cars, Faster Charging
Comprehensive Inputs on Indian EV Ecosystem
Check out the following sections for comprehensive inputs on Indian EV ecosystem (click on each section for more details)




 Our specialty focus areas include
Our specialty focus areas include